[PDF] Design And Analysis Of Clinical Experiments Ebook
Download The Design And Analysis Of Clinical Experiments 1999
Clinical study design - Wikipedia Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research (e.g., epidemiological) involving human beings. The goal of a clinical study is to assess the safety, efficacy, and / or the mechanism of action of an investigational medicinal product or procedure, or new drug or device that is in development ... Category:Design of experiments - Wikipedia Experimental design is the design of all information-gathering exercises where variation is present, whether under the full control of the experimenter or an observational study.The experimenter may be interested in the effect of some intervention or treatment on the subjects in the design. Subcategories. This category has the following 4 subcategories, out of 4 total. Design of Experiments JMP Design of Experiments (DOE) with JMP . Design of experiments, or DOE, is a practical and ubiquitous approach for exploring multifactor opportunity spaces, and JMP offers world-class capabilities for design and analysis in a form you can easily use.

Amazon Com Principles And Techniques Of Biochemistry And
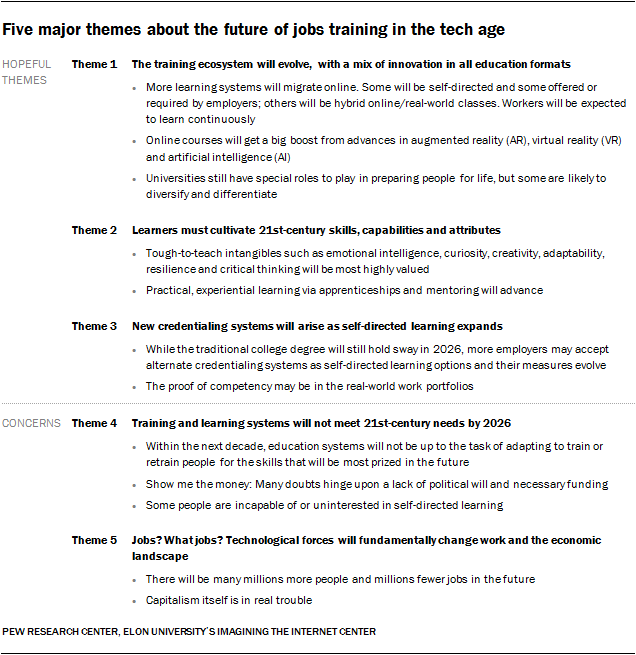
The Future Of Jobs And Education A New Pew Study Bryan
1997 Ford F250 Heavy Duty Cars For Sale
Ford F100 Cars For Sale In Lancaster California
1990 Ford F Super Duty Cars For Sale

0 Response to "Design And Analysis Of Clinical Experiments"
Post a Comment